Clinical Project Manager Resume Examples

Jul 18, 2024
|
12 min read
Nail your clinical project manager resume: A step-by-step guide to crafting a standout CV that showcases your skills, experience, and leadership in clinical trials.
Rated by 348 people
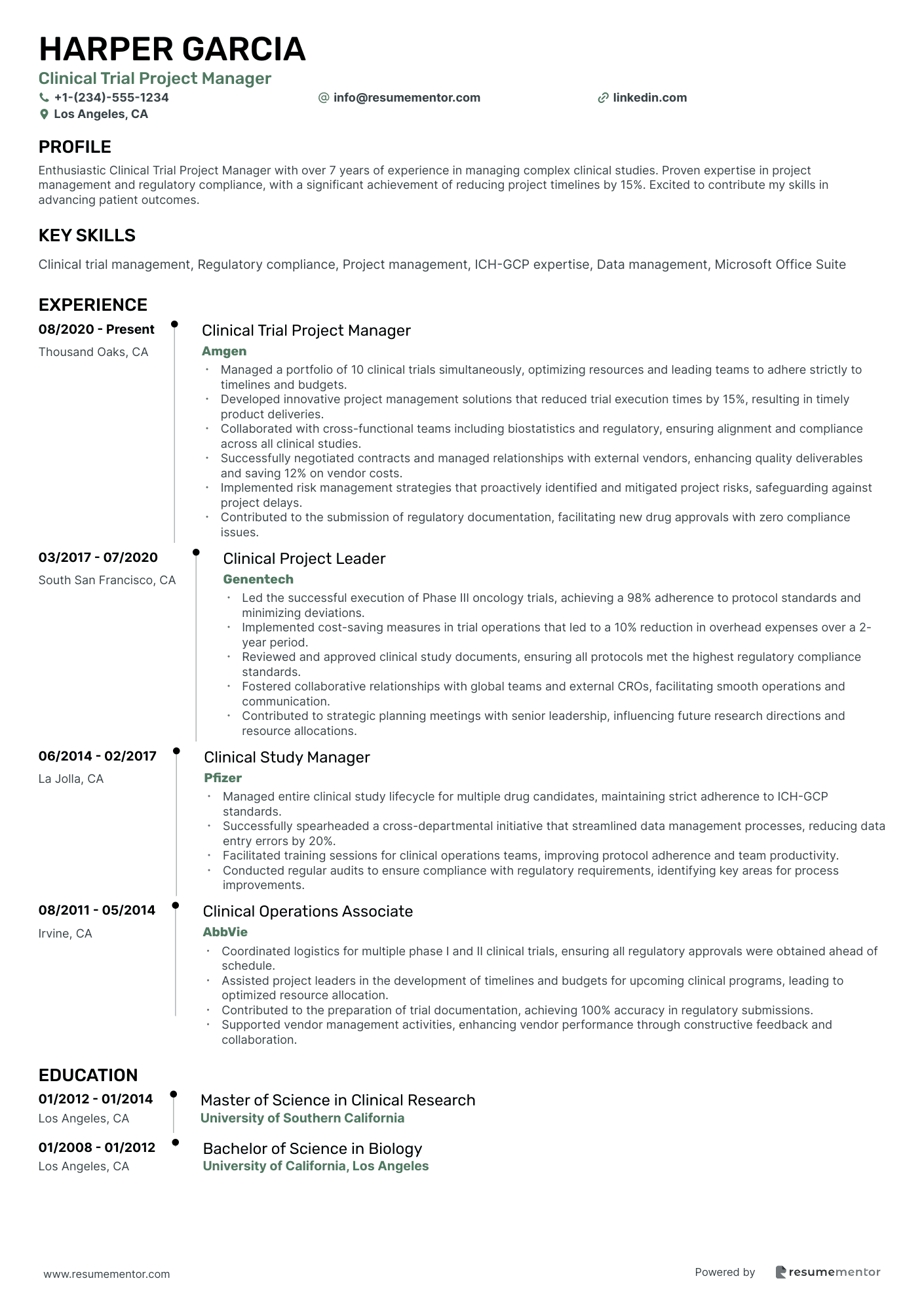
Clinical Trial Project Manager

Oncology Clinical Project Manager

Cardiology Clinical Project Manager

Pediatric Clinical Project Manager
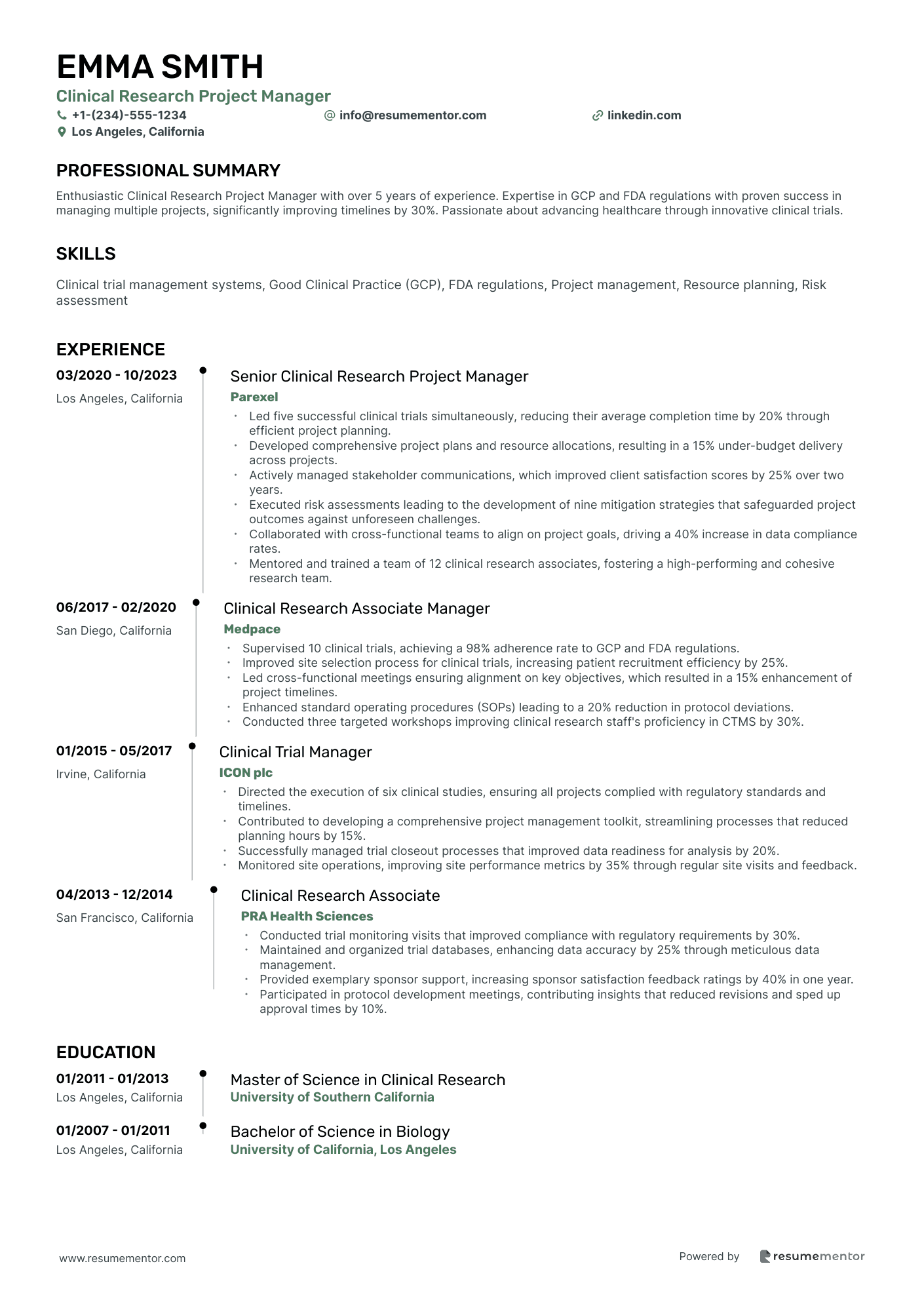
Clinical Research Project Manager

Neurology Clinical Project Manager
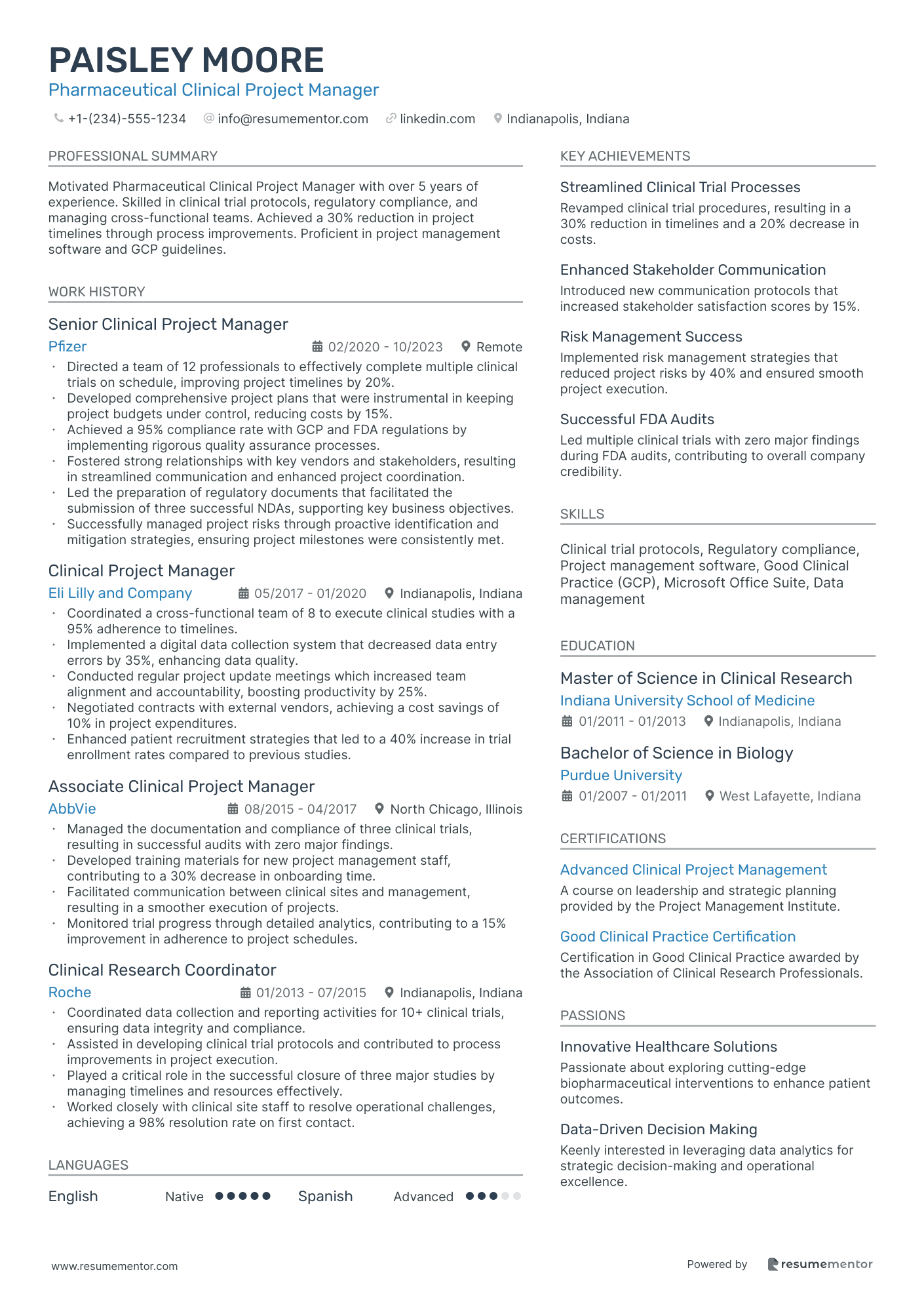
Pharmaceutical Clinical Project Manager

Medical Device Clinical Project Manager

Biotechnology Clinical Project Manager

Radiology Clinical Project Manager
Clinical Trial Project Manager resume sample
- •Managed a portfolio of 10 clinical trials simultaneously, optimizing resources and leading teams to adhere strictly to timelines and budgets.
- •Developed innovative project management solutions that reduced trial execution times by 15%, resulting in timely product deliveries.
- •Collaborated with cross-functional teams including biostatistics and regulatory, ensuring alignment and compliance across all clinical studies.
- •Successfully negotiated contracts and managed relationships with external vendors, enhancing quality deliverables and saving 12% on vendor costs.
- •Implemented risk management strategies that proactively identified and mitigated project risks, safeguarding against project delays.
- •Contributed to the submission of regulatory documentation, facilitating new drug approvals with zero compliance issues.
- •Led the successful execution of Phase III oncology trials, achieving a 98% adherence to protocol standards and minimizing deviations.
- •Implemented cost-saving measures in trial operations that led to a 10% reduction in overhead expenses over a 2-year period.
- •Reviewed and approved clinical study documents, ensuring all protocols met the highest regulatory compliance standards.
- •Fostered collaborative relationships with global teams and external CROs, facilitating smooth operations and communication.
- •Contributed to strategic planning meetings with senior leadership, influencing future research directions and resource allocations.
- •Managed entire clinical study lifecycle for multiple drug candidates, maintaining strict adherence to ICH-GCP standards.
- •Successfully spearheaded a cross-departmental initiative that streamlined data management processes, reducing data entry errors by 20%.
- •Facilitated training sessions for clinical operations teams, improving protocol adherence and team productivity.
- •Conducted regular audits to ensure compliance with regulatory requirements, identifying key areas for process improvements.
- •Coordinated logistics for multiple phase I and II clinical trials, ensuring all regulatory approvals were obtained ahead of schedule.
- •Assisted project leaders in the development of timelines and budgets for upcoming clinical programs, leading to optimized resource allocation.
- •Contributed to the preparation of trial documentation, achieving 100% accuracy in regulatory submissions.
- •Supported vendor management activities, enhancing vendor performance through constructive feedback and collaboration.
Oncology Clinical Project Manager resume sample
- •Managed 6 oncology clinical trials with a budget totaling over $12 million, enhancing participant enrollment by 25%.
- •Led cross-functional teams to ensure regulatory compliance, reducing audit findings by 50% through robust quality checks.
- •Spearheaded trial innovations that reduced timelines by 10%, resulting in a $300,000 cost savings.
- •Coordinated with external vendors to improve communication efficiency, elevating satisfaction scores by 15%.
- •Conducted comprehensive training programs for trial staff, increasing protocol adherence rate from 85% to 95%.
- •Established best practice guidelines, resulting in streamlined operations and enhanced project delivery timelines.
- •Oversaw planning and execution of 4 Phase III-IV oncology studies, reaching 100% of enrollment target on schedule.
- •Directed budget forecasting and resource allocation, maintaining trials within a 5% variance from financial plans.
- •Ensured strict adherence to GCP and ICH guidelines, achieving zero major compliance issues.
- •Enhanced trial monitoring processes, reducing protocol deviation incidents by 20%.
- •Facilitated stakeholder meetings that leveraged data insights, increasing decision-making efficiency by 30%.
- •Supported project management for 5 oncology trials, incorporating innovative strategies that improved timelines by 15%.
- •Collaborated with the data management team to optimize data collection processes, achieving 98% data quality compliance.
- •Managed logistics for trial initiation and site preparation, increasing site readiness by 20%.
- •Contributed to the development of protocol amendments, enhancing trial effectiveness by incorporating the latest research.
- •Monitored clinical study sites for compliance and progress, maintaining a 94% on-time data entry rate.
- •Played a key role in training site personnel, improving data accuracy from 85% to 95%.
- •Assisted in protocol design and ethics committee submissions, accelerating approval process by 2 weeks.
- •Coordinated cross-functional team efforts, resulting in the successful launch of a groundbreaking oncology treatment.
Cardiology Clinical Project Manager resume sample
- •Managed a team of 10 professionals to successfully complete a major clinical trial, resulting in actionable insights that improved cardiac treatment protocols by 15%.
- •Championed the implementation of a new data management system, reducing data entry errors by 25% and increasing efficiency in data analysis.
- •Collaborated with regulatory affairs to ensure adherence to Good Clinical Practice (GCP), resulting in zero compliance issues across four consecutive audits.
- •Developed and managed a budget of $5 million, maintaining expenditures within 3% of budgetary constraints while achieving all project deliverables.
- •Led the protocol development for a groundbreaking cardiovascular study, increasing participant enrollment rates by 20% through targeted outreach.
- •Facilitated weekly cross-functional meetings to discuss project progress, resulting in a 40% reduction in project timeline deviations.
- •Supervised 15 clinical trials from initiation through completion, achieving a 98% success rate in meeting project milestones.
- •Identified and mitigated project risks, resulting in a 20% reduction in unforeseen project delays and a smooth trial execution.
- •Managed communication with key stakeholders, ensuring that all project updates were delivered in a timely manner and understood by all parties.
- •Played a pivotal role in reducing trial operation costs by 10% through strategic vendor negotiations and resource allocations.
- •Mentored junior staff in best practices of project management, leading to a 30% increase in team productivity and project throughput.
- •Guided a team of project managers in the execution of multiple clinical trials, ensuring all phases were delivered on schedule.
- •Contributed to the development of trial protocols that improved compliance rates by 18% across several clinical sites.
- •Liaised with biostatistics and medical affairs to ensure accurate data analysis and interpreted findings for senior management.
- •Implemented corrective actions for clinical trials falling behind schedule, resulting in a rapid recovery and timely trial completion.
- •Coordinated patient recruitment and data collection for cardiology trials, resulting in a 90% enrollment completion rate.
- •Ensured trial documentation met FDA and GCP standards, leading to successful trial approval for three studies.
- •Trained new staff on clinical trial procedures and protocols, improving team efficiency by 25%.
- •Developed site-specific strategies for data collection, contributing to a 10% increase in data accuracy.
Pediatric Clinical Project Manager resume sample
- •Led a team of 10 professionals in managing three pediatric clinical trials simultaneously, reducing timelines by 20% while adhering to quality standards.
- •Successfully managed a project with a budget of $1.2 million, maintaining costs within 5% of proposed budget through strategic planning.
- •Collaborated with regulatory teams to ensure all trials met FDA and GCP guidelines, resulting in zero compliance issues during audits.
- •Developed comprehensive project plans and timelines, enhancing project tracking efficiency by 35% across all trial stages.
- •Facilitated communication between 15+ cross-functional team members, streamlining operations and improving data delivery times by 25%.
- •Conducted 12 site initiation and monitoring visits, bolstering site staff training and adherence to protocols.
- •Managed end-to-end execution of a critical pediatric trial involving 150 participants, resulting in decreased participant dropout rates by 15%.
- •Oversaw trial documentation accuracy, achieving 98% compliance on submitted ethics committee documentation.
- •Led risk management efforts, reducing potential trial delays by implementing proactive measures, thus stabilizing project timelines.
- •Delivered monthly progress reports to senior management, achieving 100% on-time delivery and decision-making support.
- •Coordinated with investigators and study sites to resolve operational challenges, increasing client satisfaction scores by 10%.
- •Assisted in the conduct of a multi-site pediatric trial, enrolling 85 patients ahead of schedule by leveraging robust site engagement strategies.
- •Optimized trial protocol adherence by 20% through the development of comprehensive site-specific training sessions.
- •Streamlined documentation processes, reducing time taken for trial data validation by 25%, enhancing overall data accuracy.
- •Collaborated with data management teams to improve data capture methodologies, increasing data reliability by 18%.
- •Coordinated a pediatric trial with 60+ participants, enhancing participant follow-up compliance rates by 22%.
- •Supported the development of trial-related materials, leading to improved protocol adherence and reduced site deviations by 15%.
- •Enhanced communication with research teams, resulting in more efficient resolution of trial-related queries.
- •Maintained trial documentation which resulted in a flawless regulatory review with zero major findings.
Clinical Research Project Manager resume sample
- •Led five successful clinical trials simultaneously, reducing their average completion time by 20% through efficient project planning.
- •Developed comprehensive project plans and resource allocations, resulting in a 15% under-budget delivery across projects.
- •Actively managed stakeholder communications, which improved client satisfaction scores by 25% over two years.
- •Executed risk assessments leading to the development of nine mitigation strategies that safeguarded project outcomes against unforeseen challenges.
- •Collaborated with cross-functional teams to align on project goals, driving a 40% increase in data compliance rates.
- •Mentored and trained a team of 12 clinical research associates, fostering a high-performing and cohesive research team.
- •Supervised 10 clinical trials, achieving a 98% adherence rate to GCP and FDA regulations.
- •Improved site selection process for clinical trials, increasing patient recruitment efficiency by 25%.
- •Led cross-functional meetings ensuring alignment on key objectives, which resulted in a 15% enhancement of project timelines.
- •Enhanced standard operating procedures (SOPs) leading to a 20% reduction in protocol deviations.
- •Conducted three targeted workshops improving clinical research staff's proficiency in CTMS by 30%.
- •Directed the execution of six clinical studies, ensuring all projects complied with regulatory standards and timelines.
- •Contributed to developing a comprehensive project management toolkit, streamlining processes that reduced planning hours by 15%.
- •Successfully managed trial closeout processes that improved data readiness for analysis by 20%.
- •Monitored site operations, improving site performance metrics by 35% through regular site visits and feedback.
- •Conducted trial monitoring visits that improved compliance with regulatory requirements by 30%.
- •Maintained and organized trial databases, enhancing data accuracy by 25% through meticulous data management.
- •Provided exemplary sponsor support, increasing sponsor satisfaction feedback ratings by 40% in one year.
- •Participated in protocol development meetings, contributing insights that reduced revisions and sped up approval times by 10%.
Neurology Clinical Project Manager resume sample
- •Implemented a streamlined project management process, reducing trial completion time by 15% and improving compliance with GCP standards.
- •Led a team of 8 project managers, achieving a 20% increase in successful clinical trial submissions, while maintaining high staff morale.
- •Collaborated with cross-functional teams to ensure timely delivery of 5 multi-country clinical trials, enhancing data accuracy by 25%.
- •Managed budget negotiations resulting in a cost saving of $500,000, greatly contributing to the company's operational efficiency.
- •Developed a training protocol for site initiations, reducing site setup time by 25% and enhancing staff preparedness.
- •Oversaw regulatory submissions and communication, achieving a consistent 100% submission approval rate by the first submission deadline.
- •Directed multi-national clinical trials in neurology, successfully managing over $10 million in trial budgets with precision.
- •Initiated more than 12 site selection processes per project, which effectively increased patient recruitment by 18%.
- •Fostered communication between international clinical sites and stakeholders resulting in enhanced project synchronization.
- •Executed corrective action plans for trials, reducing protocol deviations by 40% and increasing compliance.
- •Mentored junior staff and improved their project management skills, resulting in career advancements for 5 team members.
- •Managed the execution of clinical protocols, increasing trial enrollment efficiency by 20% and improving patient retention rates.
- •Coordinated with Data Management and Biostatistics teams to enhance data collection protocols, improving data accuracy by 15%.
- •Monitored site management and facilitated audits, consistently achieving positive audit outcomes with zero major deficiencies.
- •Developed comprehensive project timelines that enabled the tracking of critical milestones, contributing to a 25% reduction in timeline deviations.
- •Conducted monitoring of 15 clinical sites, ensuring adherence to protocols and compliance with ICH-GCP guidelines.
- •Coordinated with site teams to streamline processes and achieve a 10% improvement in patient follow-up compliance.
- •Participated in the design of study protocols, contributing to the successful implementation of 3 major clinical trials.
- •Supported regulatory submission processes leading to timely milestone achievements, aligning with project objectives.
Pharmaceutical Clinical Project Manager resume sample
- •Directed a team of 12 professionals to effectively complete multiple clinical trials on schedule, improving project timelines by 20%.
- •Developed comprehensive project plans that were instrumental in keeping project budgets under control, reducing costs by 15%.
- •Achieved a 95% compliance rate with GCP and FDA regulations by implementing rigorous quality assurance processes.
- •Fostered strong relationships with key vendors and stakeholders, resulting in streamlined communication and enhanced project coordination.
- •Led the preparation of regulatory documents that facilitated the submission of three successful NDAs, supporting key business objectives.
- •Successfully managed project risks through proactive identification and mitigation strategies, ensuring project milestones were consistently met.
- •Coordinated a cross-functional team of 8 to execute clinical studies with a 95% adherence to timelines.
- •Implemented a digital data collection system that decreased data entry errors by 35%, enhancing data quality.
- •Conducted regular project update meetings which increased team alignment and accountability, boosting productivity by 25%.
- •Negotiated contracts with external vendors, achieving a cost savings of 10% in project expenditures.
- •Enhanced patient recruitment strategies that led to a 40% increase in trial enrollment rates compared to previous studies.
- •Managed the documentation and compliance of three clinical trials, resulting in successful audits with zero major findings.
- •Developed training materials for new project management staff, contributing to a 30% decrease in onboarding time.
- •Facilitated communication between clinical sites and management, resulting in a smoother execution of projects.
- •Monitored trial progress through detailed analytics, contributing to a 15% improvement in adherence to project schedules.
- •Coordinated data collection and reporting activities for 10+ clinical trials, ensuring data integrity and compliance.
- •Assisted in developing clinical trial protocols and contributed to process improvements in project execution.
- •Played a critical role in the successful closure of three major studies by managing timelines and resources effectively.
- •Worked closely with clinical site staff to resolve operational challenges, achieving a 98% resolution rate on first contact.
Medical Device Clinical Project Manager resume sample
- •Spearheaded a complex cardiovascular device trial, adhering to timeline and budget constraints, achieving a 30% faster project completion rate.
- •Led a multidisciplinary team of 15 professionals, improving departmental productivity and collaboration efficacy by 25%.
- •Implemented a risk-based monitoring approach resulting in a 35% reduction in on-site monitoring visits without compromising data quality.
- •Collaborated with CROs to facilitate data analysis and report generation, contributing to regulatory approval within six months.
- •Strategized project budgets effectively, resulting in a cost-saving of 20% compared to previous trials.
- •Enhanced clinical trial documentation processes, leading to a 45% increase in regulatory submission efficiency.
- •Managed the execution of trials for a novel glucose monitoring system, increasing customer satisfaction scores by 40%.
- •Developed and managed project plans aligning with strategic goals, achieving an 85% adherence rate to scheduled timelines.
- •Conducted site selection and initiation visits, resulting in the identification and engagement of 10% more high-performing sites.
- •Enhanced cross-functional team communication protocols, reducing project delays by 30% through effective stakeholder engagement.
- •Integrated new reporting tools to streamline trial data collection, cutting data discrepancy rates by 50%.
- •Supported the setup of clinical trial sites, which resulted in a 20% increased efficiency in site activation times.
- •Assisted in the preparation of trial documentation leading to a 60% reduction in document review time.
- •Conducted on-site and remote monitoring to consistently ensure GCP adherence, resulting in zero critical findings across 15 audited sites.
- •Analyzed trial data in collaboration with the biostatistics team, enhancing report accuracy by 25%.
- •Coordinated timelines and resources for trials, yielding an 18% increase in on-time project deliveries.
- •Supported clinical project managers in implementing tracking systems, enhancing data management efficiency by 20%.
- •Facilitated communication between stakeholders, leading to improved project transparency and a 15% increase in stakeholder engagement.
- •Participated in the development of training materials, contributing to a 25% improvement in team onboarding process.
Biotechnology Clinical Project Manager resume sample
- •Led a cross-functional team in a major oncology trial, completing Phase II within a year, 20% quicker than projections.
- •Streamlined project timelines through efficient resource allocation, reducing overall trial timelines by 15%.
- •Ensured all clinical trials adhered to FDA and GCP regulations, achieving 100% compliance audit results.
- •Managed a $5 million budget with precise tracking, ultimately saving the company 10% in projected costs.
- •Implemented strategic vendor partnerships, enhancing operational efficiencies by increasing site response times by 25%.
- •Facilitated effective cross-departmental communication, resulting in a 30% improvement in stakeholder alignment.
- •Directed multiple global clinical trials, meeting all milestones and expanding the project scope by 30%.
- •Pioneered an in-house training program for regulatory compliance, improving team effectiveness by 40%.
- •Developed integrated project plans, reducing project setup time by 20% due to enhanced processes.
- •Mitigated potential risks by implementing proactive monitoring systems, resulting in 95% project success rate.
- •Managed relationships with over 20 international clinical research sites, enhancing strategic site engagement.
- •Executed high-profile clinical studies and achieved top-tier publication in recognized medical journals.
- •Ensured strict adherence to trial protocols, achieving a first-pass quality audit clearance rate of 98%.
- •Collaborated with multi-disciplinary teams and optimized data management processes, reducing errors by 25%.
- •Provided mentorship to junior associates, fostering a culture of excellence and improving team metrics.
- •Managed site recruitment and training for multiple study locations, achieving enrollment targets consistently.
- •Optimized data collection methodologies, resulting in a 30% increase in data accuracy and reliability.
- •Coordinated cross-site communications, reducing project delays by 20% through enhanced stakeholder engagement.
- •Developed site-specific monitoring reports, improving the visibility and the reporting of study progress.
Radiology Clinical Project Manager resume sample
- •Led cross-functional teams delivering radiology projects 20% faster than projected timelines, resulting in enhanced service levels.
- •Developed project plans decreasing operational budget deviations by 15%, optimizing resource allocation efficiently.
- •Facilitated regulatory compliance audits ensuring zero compliance violations over a two-year review period.
- •Implemented project management training program, improving team proficiency by 40% in six months.
- •Reduced project risk impact through innovative mitigation strategies, enhancing project outcome success rates by 25%.
- •Coordinated 50+ project meetings annually, delivering actionable insights and ensuring stakeholder accountability.
- •Supported execution of radiology initiatives saving the department $50,000 over three fiscal years.
- •Oversaw 15 radiology projects with consistent on-time delivery, achieving a 95% success rate.
- •Developed SOPs that reduced report turnaround times by 25%, enhancing radiology team workflow.
- •Facilitated communication that improved stakeholder satisfaction scores by 30% within the first quarter.
- •Implemented tracking tools that improved project status visibility and timeline adherence by 35%.
- •Managed radiology operation logistics reducing overhead costs by $10,000 annually, enhancing budget efficiency.
- •Enhanced communication frameworks increasing project awareness and timely updates by 45% within teams.
- •Optimized imaging appointment scheduling systems, improving patient satisfaction ratings by 20%.
- •Utilized project management software to enhance strategic planning processes by 30%.
- •Analyzed data improving radiology system efficiency by 15%, greatly improving operational performance.
- •Collaborated with IT teams to integrate advanced imaging technology effectively, resulting in increased usage by 20%.
- •Performed risk assessments identifying and mitigating potential project roadblocks, increasing project success rate.
- •Managed system updates decreasing downtime by 30%, ensuring continuous access to critical information.
As a clinical project manager, navigating the job market can feel like steering a ship through uncertain seas. Your resume is your vessel to new opportunities, guiding you toward a clear destination by showcasing your leadership and clinical expertise. Highlighting your ability to manage complex projects and lead teams effectively is crucial.
To make this task easier, using a reliable resume template can provide a solid foundation. It helps ensure you cover all essential components without the hassle of worrying about formatting. Check out these resume templates to find one that suits your needs and sets you on the right course.
Crafting a resume that effectively conveys your technical skills and project successes can be challenging. It's about striking the right balance between hard data, such as trial results, and softer skills like team leadership. Weaving your experiences into a compelling story is key to showcasing your achievements.
A strong template assists in organizing your information efficiently, ensuring your skills and accomplishments shine through. Since hiring managers often scan resumes quickly, clarity and structure are critical. With the right template and focus, your resume will catch their attention and open the door to your next great opportunity.
Key Takeaways
- Highlight your expertise in clinical trials, regulatory compliance, and team leadership in your clinical project manager resume.
- Use a reverse-chronological format to effectively showcase recent and relevant experiences, and choose modern fonts for readability.
- Incorporate quantifiable achievements in your experience section using strong action words to narrate your impact and accomplishments.
- Include both hard and soft skills and list relevant certifications like PMP and CCRA to strengthen your qualifications.
- Complement your resume with sections like languages, hobbies, volunteer work, and relevant books to present a well-rounded profile.
What to focus on when writing your clinical project manager resume
Your clinical project manager resume should seamlessly convey your expertise in managing clinical trials, ensuring regulatory compliance, and leading teams effectively. Start by ensuring your contact information, including your name, phone number, email, and LinkedIn profile, is easy for recruiters to find—this way, they can reach you without any hassle. This top section sets the stage for a recruiter to get in touch whenever they need more information or wish to schedule an interview.
How to structure your clinical project manager resume
- Begin the main content with a professional summary that captures the essence of your clinical research experience and leadership qualities. This brief yet comprehensive introduction sets the tone for the depth of your project management skills to follow. By summarizing your accomplishments and goals here, you make a strong first impression that encourages further reading.
- When detailing your work experience, focus on your successes in managing clinical projects and meeting strict regulations—highlight concrete accomplishments like cost reduction, improved project timelines, or successful audits. Use bullet points for clarity and incorporate metrics to make your achievements tangible. Demonstrating how you've contributed to the success of past employers adds credibility to your application.
- Your education section should naturally follow, emphasizing degrees, certifications, and coursework relevant to clinical research or project management. This serves as the foundation of your expertise, showing the academic and professional knowledge base that allows you to perform effectively in the role. Remember to highlight any honors or special programs that further bolster your qualifications.
- In your skills section, underscore attributes like clinical trial management, regulatory expertise, and leadership. These skills provide the bridge between your education and work experiences, demonstrating how you effectively navigate the complexities of clinical project management. Skills here should be pertinent and consistently reflected in other sections of your resume.
- Finally, list any certifications, such as PMP or clinical research-specific credentials like ACRP and SOCRA. These prove your ongoing commitment to staying at the forefront of your field, presenting you as a proactive learner dedicated to maintaining high standards in clinical project management.
To round things out, consider adding "Awards" or "Volunteer Experience" sections—these can enhance your profile by giving additional insight into your accomplishments and dedication. Now that we've covered the overall structure, let’s go deeper into each section and how to tailor them effectively for a clinical project manager’s success.
Which resume format to choose
Crafting a compelling resume as a clinical project manager starts with choosing the right format, and the reverse-chronological layout is ideal for this role. It naturally highlights your most recent and relevant experiences, allowing potential employers to quickly assess your current capabilities and successes.
When it comes to font choice, opting for a modern style like Lato, Montserrat, or Raleway can enhance readability while making your resume visually appealing. These fonts offer a clean, contemporary look that aligns with the professional tone you want to set.
Saving your resume as a PDF is crucial for maintaining its format across different devices. This ensures that your carefully structured information remains consistent and looks professional no matter how it's viewed by employers.
Paying attention to margins is also important. Using one-inch margins on all sides creates a balanced layout that includes plenty of white space. This makes your resume easy to read and prevents it from appearing cluttered, helping to ensure that your key achievements and skills stand out.
Together, these elements form a cohesive resume that effectively highlights your strengths as a candidate in clinical project management, presenting you as both organized and detail-oriented—qualities essential for success in this role.
How to write a quantifiable resume experience section
A clinical project manager resume should capture attention with a well-crafted experience section that flows naturally. This part narrates your professional journey by emphasizing results and skills directly related to your field. Structuring it in reverse chronological order allows potential employers to see your most recent and relevant experiences first. It's ideal to cover the last 10-15 years or focus on positions that directly pertain to the role you're targeting. Align job titles with your responsibilities to the desired job by incorporating keywords from the job ad and focusing on achievements that mirror the role's demands. Using strong action words like "led," "managed," and "achieved" effectively highlights your impact and contributions.
- •Led a cross-functional team to reduce clinical trial timelines by 20% while maintaining regulatory compliance.
- •Implemented a new project management tool that increased team productivity by 15%.
- •Developed a risk management plan that decreased project risks by 30% and improved project delivery timelines.
- •Coordinated with global teams to ensure successful trial execution, resulting in a 95% approval rate for new drug applications.
The experience section above effectively combines tailored job titles and quantifiable results that directly align with the clinical project manager role. By using strong action words and specific achievements, it seamlessly showcases your capabilities and the tangible impact you've made. Each bullet point connects your past roles to the potential employer’s needs, emphasizing your results-oriented approach. This integration creates a compelling narrative in your resume, naturally drawing attention to how you stand out as a candidate.
Customer-Focused resume experience section
A customer-focused clinical project manager resume experience section should clearly demonstrate how you build and maintain strong relationships with clients and stakeholders. Start by detailing how your efforts lead to positive outcomes, using clear language and measurable results that illustrate your impact. Highlight your ability to understand and anticipate clients' needs, which helps foster trust and create long-lasting partnerships.
To make your achievements stand out, emphasize skills like communication, leadership, and problem-solving through dynamic action verbs. Instead of simply mentioning "Managed project schedules," describe how you "Led the management of project schedules, ensuring timely delivery," which underscores a proactive approach. Keep the focus on how you consistently meet customer needs, add value to projects, and cultivate beneficial relationships that enhance success.
Clinical Project Manager
HealthTech Solutions
June 2018 - Present
- Led 12+ clinical trials by coordinating with cross-functional teams, delivering results on-time with 95% customer satisfaction rating.
- Implemented a feedback system to identify client needs, improving service delivery and increasing repeat business by 30%.
- Collaborated with clients to understand specific project goals, consistently exceeding expectations and enhancing client trust.
- Redesigned client onboarding process, reducing time to onboarding by 40% and improving initial client satisfaction scores.
Growth-Focused resume experience section
A Growth-Focused clinical project manager resume experience section should clearly demonstrate how you have actively contributed to a company’s progress. Begin by offering examples of projects where your leadership directly led to business growth, seamlessly transitioning into discussing the tangible benefits, such as cost savings and increased efficiency, you achieved through innovative strategies. Highlight your collaborative efforts with other departments, as these are crucial in ensuring projects not only align with but also support the company’s broader objectives, showcasing your strong strategic insight.
Throughout your descriptions, use straightforward language to convey your leadership and decision-making expertise, grounding your claims with quantifiable metrics like numbers and percentages. This approach will appeal to prospective employers looking for professionals who can drive results and support organizational growth. By weaving these elements together, you illustrate that you understand the larger goals of the company and are equipped to meaningfully contribute to its success.
Clinical Project Manager
HealthTech Solutions Inc.
2019 - 2022
- Redesigned clinical study protocols, shortening timelines by 20% and saving $500,000 annually, enhancing project efficiency.
- Worked with cross-functional teams to streamline workflows, leading to a 15% increase in study participant retention, which strengthened project outcomes.
- Adopted a new data management system, boosting data accuracy by 25% and reducing processing time by 30%, improving overall project quality.
- Led strategic planning to ensure clinical projects aligned with company growth targets, resulting in a 10% rise in annual revenue and demonstrating scalable impact.
Responsibility-Focused resume experience section
A responsibility-focused clinical project manager resume experience section should highlight the impact you’ve made in your roles through clear and connected narratives. Start by detailing the key responsibilities you managed and the projects you led. Use straightforward language and provide examples that showcase your contributions to successful outcomes. Include figures to demonstrate how you introduced innovative processes, managed budgets, and led teams through complex projects, tying these achievements together to show your overall effectiveness.
Your goal is to create a cohesive story of your achievements and skills that is both clear and engaging. Quantifying your successes, such as how much you reduced project timelines or increased budget efficiency, further strengthens your narrative. By offering concrete examples of your leadership and strategic planning abilities, you can help potential employers understand your value. Make sure each bullet point builds on this story without overwhelming it with technical jargon or unnecessary detail.
Senior Clinical Project Manager
Health Innovations Inc.
June 2018 - Present
- Led a cross-functional team of 15 to complete Phase III clinical trials, reducing time-to-market by 20%.
- Implemented new project tracking software, improving budget management accuracy by 25%.
- Coordinated with external vendors, enhancing communication strategies to cut project overhead by 10%.
- Developed training programs for new hires, boosting their productivity by 30%.
Achievement-Focused resume experience section
A clinical project manager achievement-focused resume experience section should effectively highlight the impact you've made in your previous roles, showcasing your ability to drive success. Begin by highlighting specific accomplishments rather than listing your daily tasks. Use measurable results, action verbs, and clear metrics to underscore your contributions. This approach not only makes your skills more apparent but also allows potential employers to quickly assess your fit for the position.
Ensure your achievements are listed in order of relevance and significance, tailored to the job you're applying for. A clear, concise structure helps avoid overwhelming detail, allowing your successes to shine. Focus on demonstrating your strengths in managing complex projects, leading diverse teams, and achieving significant results. By maintaining straightforward and cohesive language, you can create a powerful narrative that captures the attention of hiring managers.
Senior Clinical Project Manager
MediCorp Solutions
June 2018 – Present
- Led a cross-functional team of 20+ in completing a clinical trial two months ahead of schedule, saving $200,000.
- Implemented a new project management software, boosting team efficiency by 30% and improving data tracking accuracy.
- Coordinated with external vendors and stakeholders to improve communication, cutting project delays by 25%.
- Developed and trained junior project managers, enhancing team capability and promoting three to senior roles.
Write your clinical project manager resume summary section
A results-focused clinical project manager resume summary should concisely capture the essence of your career in just a few sentences. Start by highlighting your job title and years of experience to establish credibility. Then, move on to an achievement that underscores your skills. For instance, consider this example:
This summary effectively showcases your background, highlights a quantifiable success, and summarizes your key skills in a way that is impactful. Tailoring it with specifics from job postings can further enhance how you stand out. Describing yourself well includes using action verbs to show impact, like "improving" or "leading," integrating seamlessly with your achievement narrative. While a summary gives an overview of your career, a resume objective often outlines future goals, such as "seeking to leverage my skills…". In contrast, a resume profile might focus on your current responsibilities, while a summary of qualifications offers a bullet-point list of your top strengths. Understanding these nuances helps you showcase your experience more effectively, ensuring your resume captures the reader's attention.
Listing your clinical project manager skills on your resume
A skills-focused clinical project manager resume should seamlessly integrate both hard and soft skills to present a well-rounded candidate profile. While listing your skills as a standalone section is effective, weaving them into your experience and summary sections can also highlight your strengths. Soft skills, such as communication and leadership, are personal traits that facilitate teamwork and collaboration. Meanwhile, hard skills refer to specific abilities or knowledge, like proficiency in clinical trial management software, that are vital for the technical demands of the role.
Incorporating these skills and strengths into your entire resume not only shows your qualifications clearly but also helps align your profile with job descriptions. This alignment is crucial for ensuring your resume gets noticed by employers and passes through applicant tracking systems effectively.
This example is impactful due to its clarity and focus, highlighting the essential skills necessary for a clinical project manager. Listing eight targeted skills demonstrates a precise expertise, while using industry-standard terms like "Regulatory Compliance" and "Clinical Data Analysis" provides a clear picture of your capabilities, making it easy for recruiters to recognize your strengths.
Best hard skills to feature on your clinical project manager resume
Hard skills for a clinical project manager should reflect the technical expertise required and showcase your ability to manage clinical trials successfully.
Hard Skills
- Clinical Trial Management
- Regulatory Compliance
- Clinical Research Regulations (ICH-GCP, FDA)
- Clinical Data Management
- Budget Planning and Management
- Risk Assessment and Management
- Quality Assurance
- Time Management Software Proficiency
- Project Scheduling
- Biostatistics
- Scientific Writing
- Clinical Software Platforms (e.g., EDC, CTMS)
- Vendor Management
- SOP Development
- Medical Writing
Best soft skills to feature on your clinical project manager resume
Soft skills should highlight your capability to lead teams and effectively communicate in your role as a clinical project manager.
Soft Skills
- Leadership
- Communication
- Problem-Solving
- Negotiation
- Team Building
- Adaptability
- Decision Making
- Critical Thinking
- Detail Orientation
- Emotional Intelligence
- Conflict Resolution
- Motivational Skills
- Organizational Skills
- Interpersonal Skills
- Time Management
How to include your education on your resume
The education section is an essential part of your resume as a clinical project manager. It showcases your academic background and helps demonstrate your knowledge and skills in the field. Tailor this section to the job you're applying for and exclude any irrelevant education that doesn't support your candidacy. Include your GPA if it's strong and relevant; typically, a GPA of 3.5 or higher is worth mentioning. For honors like cum laude, include them to highlight your academic achievements. When listing your degree, use the full title followed by the concentration, if applicable.
Here’s a wrong example:
Here’s a right example:
- •Graduated cum laude
The second example is effective because it highlights a relevant degree for the clinical project manager role. It includes a strong GPA and honors, which reinforce academic excellence. Omitting the location for the institution helps keep the focus on pertinent details. The dates show a realistic timeline for obtaining the degree, adding credibility. This example paints a clear picture of an educated and capable candidate ready for the role.
How to include clinical project manager certificates on your resume
Certifications play an essential role in a clinical project manager's resume, demonstrating your skills and professional development. To effectively include this section, list the name of each certification clearly. Include the date when you received the certification. Add the issuing organization for credibility. You can also feature your key certificates in the header of your resume for quick reference.
For example, you could have your name as "Jane Doe, PMP, CCRA" right at the top of your resume. Highlighting your most relevant certifications up front immediately shows your qualifications. A detailed certification section gives potential employers confidence in your abilities and dedication to continuous learning.
This example is effective because it lists relevant certifications like PMP and CCRA, which are crucial for a clinical project manager. The issuing organizations, Project Management Institute and ACRP, add credibility. Dates are recommended to be included but are omitted here for simplicity.
Extra sections to include in your clinical project manager resume
When crafting a resume for a clinical project manager, including various sections can make your profile well-rounded and appealing to employers. Each section serves a unique purpose and provides a broader picture of your skills, character, and qualifications.
Language — List any languages you speak fluently to show your capability for global communication. This skill can give you an edge in multinational projects.
Hobbies and interests — Add hobbies that relate to leadership or analytical skills, such as chess or hiking. This section humanizes you and shows a balanced approach to work and life.
Volunteer work — Highlight your volunteer efforts, especially in healthcare or project management settings. Demonstrate your commitment to social causes and your willingness to go above and beyond.
Books — Mention relevant books you have read about project management or clinical research. This shows that you are continually learning and staying updated in your field.
In Conclusion
In conclusion, crafting an effective clinical project manager resume requires a strategic approach that showcases your leadership, accomplishments, and relevant skills. Your resume serves as a gateway to new opportunities, highlighting your ability to manage complex projects and navigate the intricacies of clinical trials. By using a reliable resume template, you ensure that all vital components are presented clearly and professionally. The right format, such as a reverse-chronological layout, will help hiring managers quickly assess your recent experiences and qualifications.
In your resume, integrate quantifiable results and action verbs to emphasize your impact in past roles, and tailor each section to the job description. Include both hard and soft skills to present a comprehensive view of your capabilities. Skills in clinical trial management, regulatory compliance, and effective communication are essential for success in this role. Additionally, featuring a strong educational background and relevant certifications will reinforce your expertise and commitment to continuous professional development.
Remember to add any extra sections that can set you apart, such as language skills, hobbies related to leadership or analytical thinking, and volunteer work in healthcare settings. These elements capture a broader picture of who you are as a candidate. Overall, a well-structured resume that effectively balances technical and interpersonal skills will position you as a strong candidate for clinical project management positions, ready to embrace new challenges and drive impactful results.
Related Articles

Continue Reading
Check more recommended readings to get the job of your dreams.
Resume
Resources
Tools
© 2026. All rights reserved.
Made with love by people who care.

