Pharmaceutical Project Manager Resume Examples

Jul 18, 2024
|
12 min read
Get your dream job as a pharmaceutical project manager: A step-by-step guide to drafting a killer resume that showcases your skills and experience without any side effects.
Rated by 348 people
Clinical Trials Project Manager

Oncology Pharmaceutical Project Manager
Medical Device Project Manager

Regulatory Affairs Project Manager
Pharmaceutical Research Project Manager

Drug Discovery Project Manager

Biotech Pharmaceutical Project Manager

Pharmaceutical Project Planning Manager

Quality Assurance Project Manager in Pharma
Pharmaceutical Supply Chain Project Manager
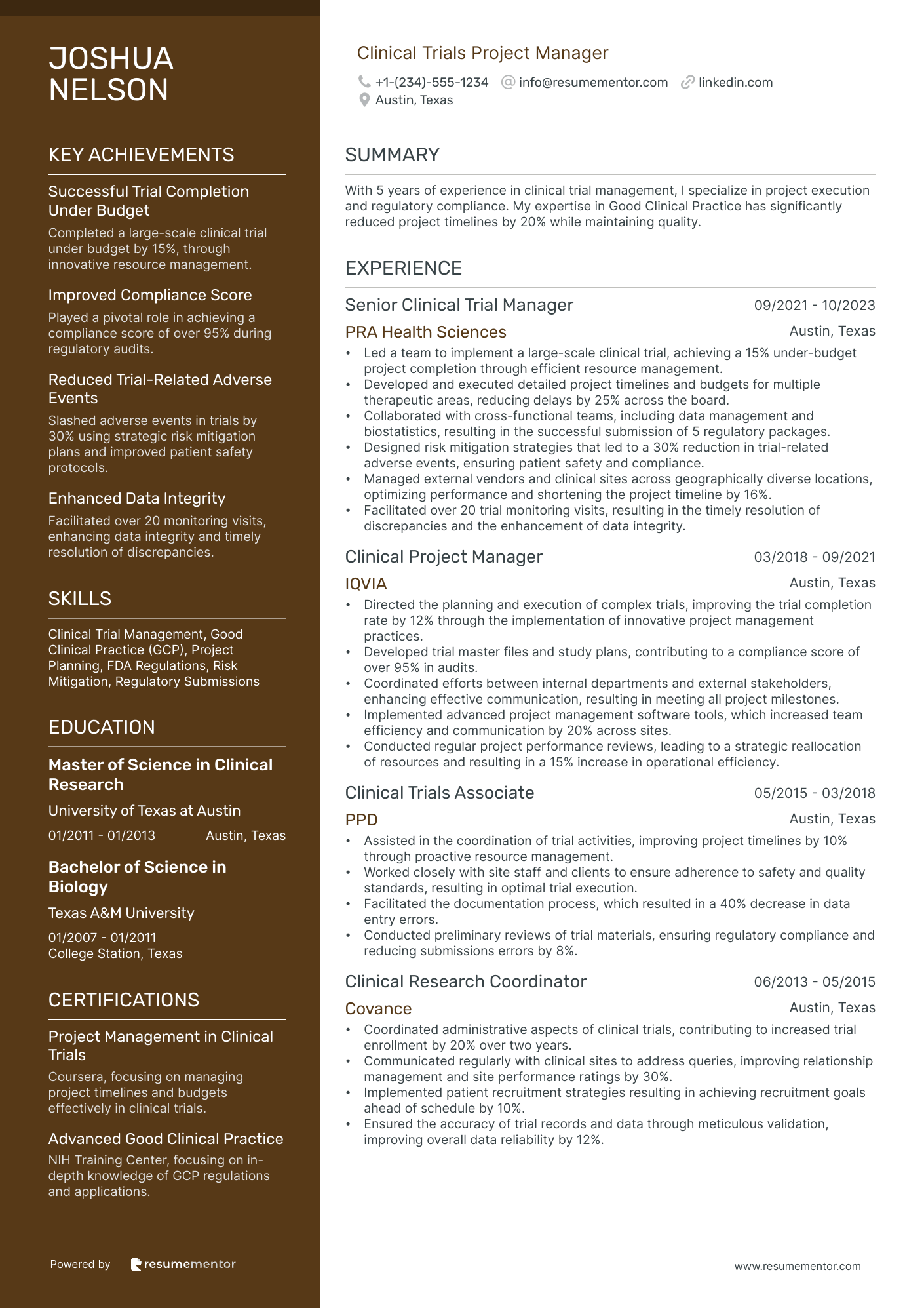
Clinical Trials Project Manager resume sample
- •Led a team to implement a large-scale clinical trial, achieving a 15% under-budget project completion through efficient resource management.
- •Developed and executed detailed project timelines and budgets for multiple therapeutic areas, reducing delays by 25% across the board.
- •Collaborated with cross-functional teams, including data management and biostatistics, resulting in the successful submission of 5 regulatory packages.
- •Designed risk mitigation strategies that led to a 30% reduction in trial-related adverse events, ensuring patient safety and compliance.
- •Managed external vendors and clinical sites across geographically diverse locations, optimizing performance and shortening the project timeline by 16%.
- •Facilitated over 20 trial monitoring visits, resulting in the timely resolution of discrepancies and the enhancement of data integrity.
- •Directed the planning and execution of complex trials, improving the trial completion rate by 12% through the implementation of innovative project management practices.
- •Developed trial master files and study plans, contributing to a compliance score of over 95% in audits.
- •Coordinated efforts between internal departments and external stakeholders, enhancing effective communication, resulting in meeting all project milestones.
- •Implemented advanced project management software tools, which increased team efficiency and communication by 20% across sites.
- •Conducted regular project performance reviews, leading to a strategic reallocation of resources and resulting in a 15% increase in operational efficiency.
- •Assisted in the coordination of trial activities, improving project timelines by 10% through proactive resource management.
- •Worked closely with site staff and clients to ensure adherence to safety and quality standards, resulting in optimal trial execution.
- •Facilitated the documentation process, which resulted in a 40% decrease in data entry errors.
- •Conducted preliminary reviews of trial materials, ensuring regulatory compliance and reducing submissions errors by 8%.
- •Coordinated administrative aspects of clinical trials, contributing to increased trial enrollment by 20% over two years.
- •Communicated regularly with clinical sites to address queries, improving relationship management and site performance ratings by 30%.
- •Implemented patient recruitment strategies resulting in achieving recruitment goals ahead of schedule by 10%.
- •Ensured the accuracy of trial records and data through meticulous validation, improving overall data reliability by 12%.
Oncology Pharmaceutical Project Manager resume sample
- •Led a project team of 12 in the successful launch of an oncology drug, achieving a 20% under-budget performance.
- •Developed risk mitigation strategies, reducing potential project delays by 15% and ensuring compliance with all regulations.
- •Coordinated with R&D, regulatory, and marketing departments to enhance inter-departmental communication, resulting in a 25% increase in project efficiency.
- •Facilitated training and mentoring for project associates, fostering a team learner culture and increasing productivity by 18%.
- •Implemented a new project management software, which improved timeline adherence by 22% across different projects.
- •Managed a comprehensive project budget of $5M, reporting financial performance on a quarterly basis to senior stakeholders.
- •Executed a strategic project plan for a novel oncology treatment, bringing the product to the market six months earlier than anticipated.
- •Balanced multiple oncology projects, achieving a 30% reduction in waste expenditure by adopting lean project practices.
- •Established a collaboration protocol with external CROs, improving project development timelines by 12% during peak phases.
- •Conducted bi-weekly project status meetings, ensuring all stakeholders were informed, which boosted project transparency by 40%.
- •Initiated a cross-departmental knowledge-sharing platform that increased regulatory compliance rate by 35% with updated industry insights.
- •Managed clinical trial operations for oncology projects, achieving a patient recruitment success rate improvement of 28%.
- •Streamlined data management processes, enhancing data accuracy by 32% for regulatory submissions within stringent timelines.
- •Oversaw a clinical team of 10, leading to improved scope adherence by 25% through effective delegation and oversight.
- •Instituted cost-control measures leading to project cost reduction of 18% without compromising quality or regulatory adherence.
- •Supported project managers in the planning and execution of oncology projects, resulting in a 15% increase in project timelines adherence.
- •Administered project documentation processes, improving compliance documentation accuracy by 20% with structured checks.
- •Facilitated team meetings, creating efficiency in scheduling and enhancing collaborative outputs by streamlining communication paths.
- •Assisted in the management of project budgets, contributing to a reduction in variance reporting by 17% through detailed tracking.
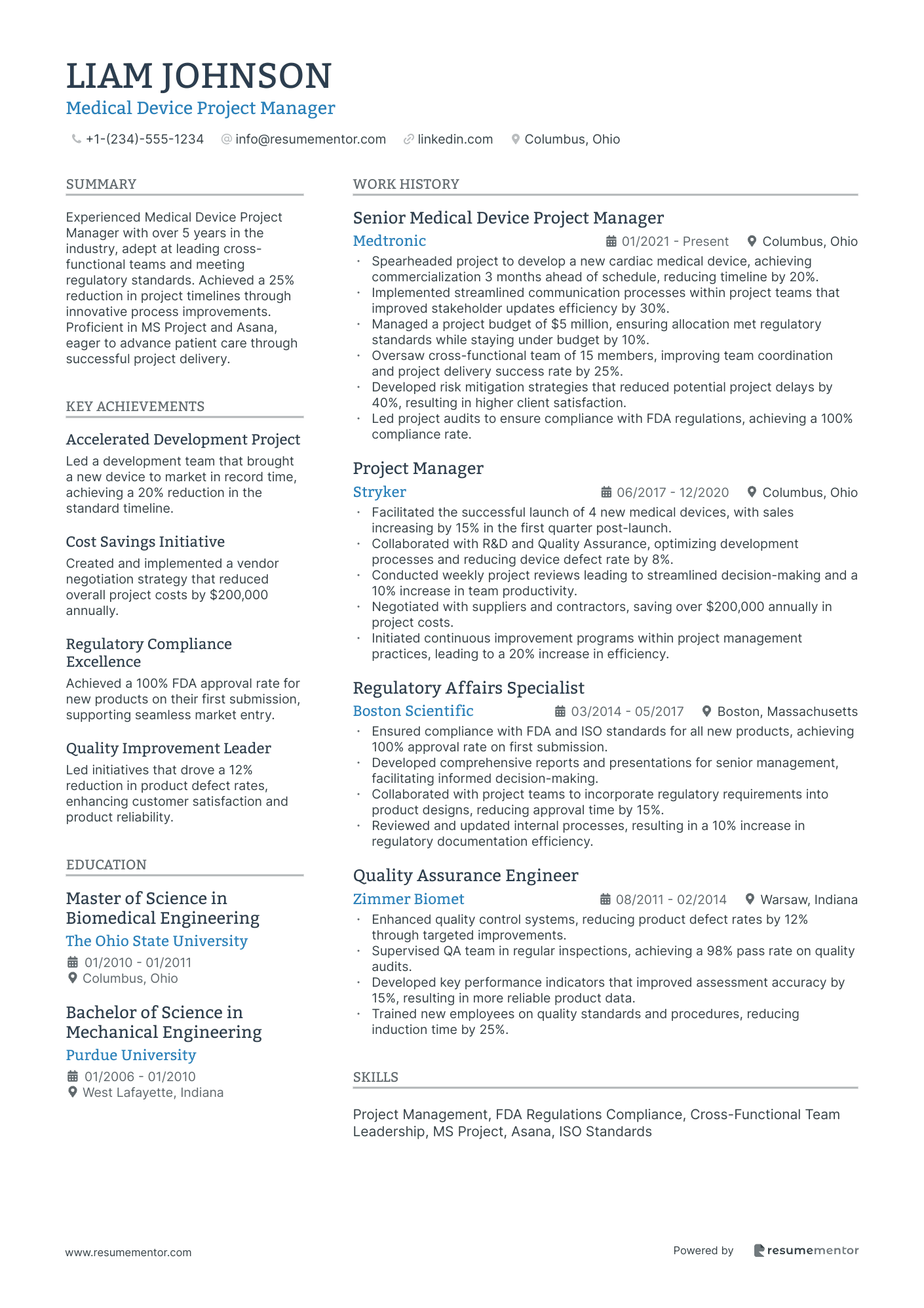
Medical Device Project Manager resume sample
- •Spearheaded project to develop a new cardiac medical device, achieving commercialization 3 months ahead of schedule, reducing timeline by 20%.
- •Implemented streamlined communication processes within project teams that improved stakeholder updates efficiency by 30%.
- •Managed a project budget of $5 million, ensuring allocation met regulatory standards while staying under budget by 10%.
- •Oversaw cross-functional team of 15 members, improving team coordination and project delivery success rate by 25%.
- •Developed risk mitigation strategies that reduced potential project delays by 40%, resulting in higher client satisfaction.
- •Led project audits to ensure compliance with FDA regulations, achieving a 100% compliance rate.
- •Facilitated the successful launch of 4 new medical devices, with sales increasing by 15% in the first quarter post-launch.
- •Collaborated with R&D and Quality Assurance, optimizing development processes and reducing device defect rate by 8%.
- •Conducted weekly project reviews leading to streamlined decision-making and a 10% increase in team productivity.
- •Negotiated with suppliers and contractors, saving over $200,000 annually in project costs.
- •Initiated continuous improvement programs within project management practices, leading to a 20% increase in efficiency.
- •Ensured compliance with FDA and ISO standards for all new products, achieving 100% approval rate on first submission.
- •Developed comprehensive reports and presentations for senior management, facilitating informed decision-making.
- •Collaborated with project teams to incorporate regulatory requirements into product designs, reducing approval time by 15%.
- •Reviewed and updated internal processes, resulting in a 10% increase in regulatory documentation efficiency.
- •Enhanced quality control systems, reducing product defect rates by 12% through targeted improvements.
- •Supervised QA team in regular inspections, achieving a 98% pass rate on quality audits.
- •Developed key performance indicators that improved assessment accuracy by 15%, resulting in more reliable product data.
- •Trained new employees on quality standards and procedures, reducing induction time by 25%.
Regulatory Affairs Project Manager resume sample
- •Spearheaded regulatory submission processes for three IND applications that received approval in under 6 months.
- •Developed comprehensive regulatory strategies that accelerated product launch timelines by 25% and enhanced market penetration.
- •Enhanced communication protocols with the FDA, resulting in a 15% reduction in review cycle times.
- •Coordinated a cross-functional team of 12 to streamline the regulatory submission process, improving efficiency by 20%.
- •Led risk assessments that identified regulatory roadblocks, enabling proactive revisions to submission strategies.
- •Authored 30+ regulatory documents, ensuring thorough compliance with all relevant regulations and guidelines.
- •Managed end-to-end submission process for an NDA, achieving approval ahead of schedule, contributing to a $20 million revenue increase.
- •Collaborated with R&D and clinical teams to identify regulatory pathways, achieving strategic alignment on project goals.
- •Conducted detailed regulatory risk assessments that informed executive decision-making, mitigating potential compliance issues.
- •Monitored legislative changes across US and EU markets, advising senior leadership on strategic implications.
- •Enhanced regulatory submission tools, resulting in a 10% increase in submission accuracy and completeness.
- •Assisted in the preparation and filing of a BLA with a major regulatory body, resulting in market approval 3 months ahead of projections.
- •Developed a tracking system for regulatory documentation, improving retrieval speed by 30% and minimizing submission errors.
- •Built relationships with regulatory personnel, facilitating smoother communication and expediting issue resolution by 20%.
- •Supported senior managers by preparing detailed analyses of regulatory guidelines, aiding in strategic planning.
- •Conducted regulatory submission reviews, ensuring compliance and facilitating faster approvals by reducing re-submission rates.
- •Tracked over 100 regulatory submissions, ensuring deadlines were met and improving department responsiveness by 15%.
- •Established a comprehensive database for regulatory documentation, enhancing cross-departmental access and decreasing information retrieval times.
- •Assisted in the implementation of eCTD standards, contributing to a 20% reduction in submission preparation times.
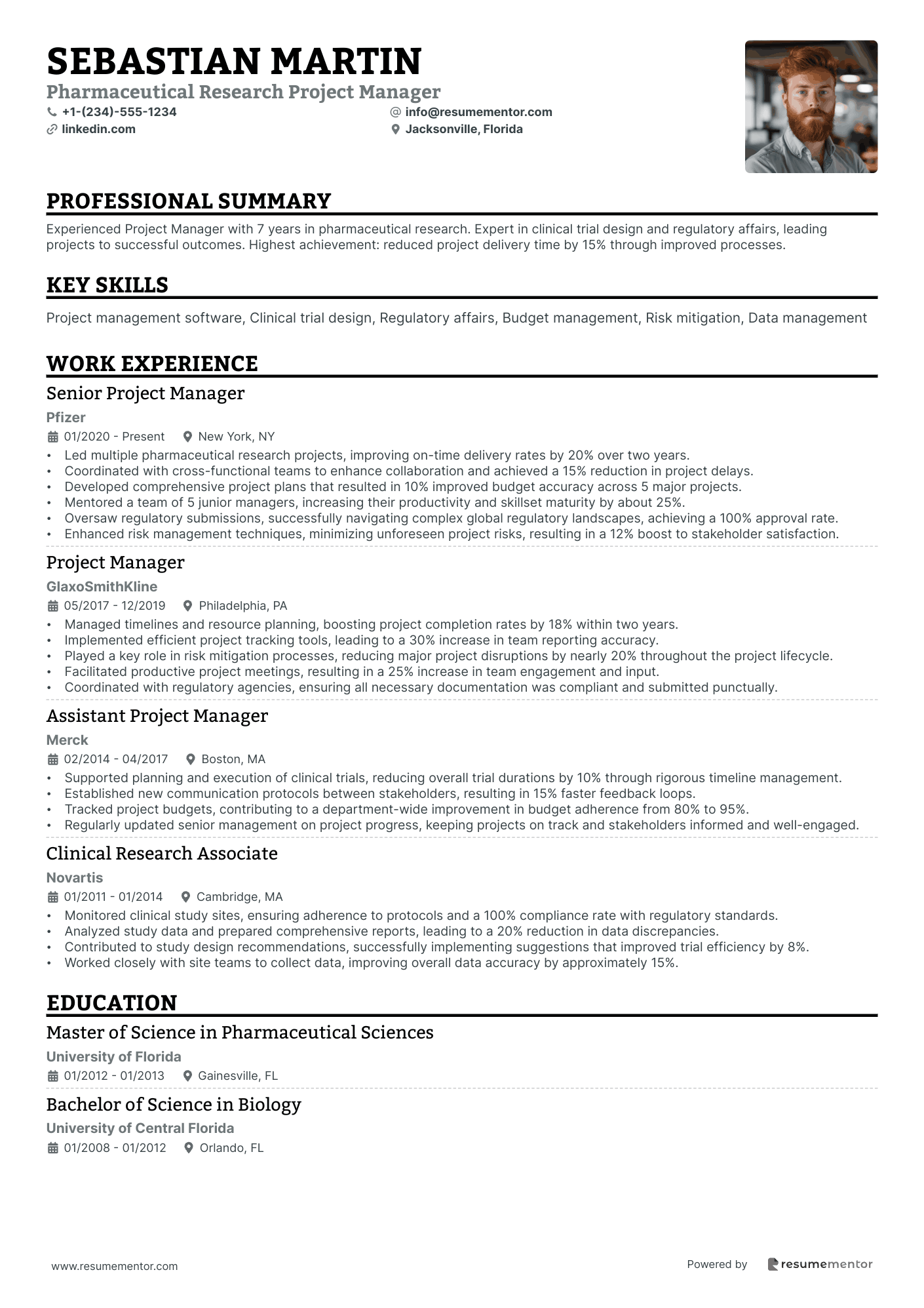
Pharmaceutical Research Project Manager resume sample
- •Led multiple pharmaceutical research projects, improving on-time delivery rates by 20% over two years.
- •Coordinated with cross-functional teams to enhance collaboration and achieved a 15% reduction in project delays.
- •Developed comprehensive project plans that resulted in 10% improved budget accuracy across 5 major projects.
- •Mentored a team of 5 junior managers, increasing their productivity and skillset maturity by about 25%.
- •Oversaw regulatory submissions, successfully navigating complex global regulatory landscapes, achieving a 100% approval rate.
- •Enhanced risk management techniques, minimizing unforeseen project risks, resulting in a 12% boost to stakeholder satisfaction.
- •Managed timelines and resource planning, boosting project completion rates by 18% within two years.
- •Implemented efficient project tracking tools, leading to a 30% increase in team reporting accuracy.
- •Played a key role in risk mitigation processes, reducing major project disruptions by nearly 20% throughout the project lifecycle.
- •Facilitated productive project meetings, resulting in a 25% increase in team engagement and input.
- •Coordinated with regulatory agencies, ensuring all necessary documentation was compliant and submitted punctually.
- •Supported planning and execution of clinical trials, reducing overall trial durations by 10% through rigorous timeline management.
- •Established new communication protocols between stakeholders, resulting in 15% faster feedback loops.
- •Tracked project budgets, contributing to a department-wide improvement in budget adherence from 80% to 95%.
- •Regularly updated senior management on project progress, keeping projects on track and stakeholders informed and well-engaged.
- •Monitored clinical study sites, ensuring adherence to protocols and a 100% compliance rate with regulatory standards.
- •Analyzed study data and prepared comprehensive reports, leading to a 20% reduction in data discrepancies.
- •Contributed to study design recommendations, successfully implementing suggestions that improved trial efficiency by 8%.
- •Worked closely with site teams to collect data, improving overall data accuracy by approximately 15%.
Drug Discovery Project Manager resume sample
- •Successfully reduced project timelines by 25% by implementing efficient project management strategies, directly contributing to accelerated drug development cycle.
- •Led a cross-functional team of 20+ scientists and researchers to achieve significant milestones, including a new candidate reaching preclinical trials within 18 months.
- •Identified and mitigated key project risks using root cause analysis, resulting in the successful prevention of potential setbacks and resource wastage.
- •Developed comprehensive project plans and budgets for projects exceeding $10 million, ensuring resources were allocated effectively and milestones met.
- •Facilitated transparent communication channels between project stakeholders, enhancing decision-making efficiency and stakeholder satisfaction.
- •Mentored team members, improving their project management skills, resulting in a 40% improvement in their individual task efficiency.
- •Delivered multiple drug discovery projects on time by developing and implementing robust risk management strategies.
- •Coordinated efforts of chemistry, biology, and regulatory teams to successfully advance projects from early-stage discovery to preclinical development.
- •Standardized project management processes that improved departmental efficiency by 30%, contributing to streamlined operations across teams.
- •Authored detailed status reports and presented project progress to executive leadership, facilitating data-driven strategic decisions.
- •Implemented industry compliance practices, ensuring all project activities adhered to the latest regulations and quality standards.
- •Co-managed a portfolio of drug discovery projects with a total budget of $15 million, achieving all project milestones on schedule.
- •Devised effective communication strategies between diverse project teams, resulting in a 25% increase in intra-team collaboration.
- •Introduced agile project management tools, which enhanced project tracking accuracy and accountability among team members.
- •Drove compliance and maintained quality standards across projects, proactively addressing regulatory issues before they arose.
- •Conducted research focused on identifying novel therapeutic targets, resulting in two patents for innovative drug candidates.
- •Collaborated with interdisciplinary team to enhance drug efficacy, resulting in improved clinical trial outcomes.
- •Optimized laboratory operations, reducing research cycle times by 20% and significantly increasing overall productivity.
- •Authored and contributed to multiple peer-reviewed publications, sharing valuable findings with the scientific community.
Biotech Pharmaceutical Project Manager resume sample
- •Led cross-functional teams to advance a complex drug development project from Phase II to Phase III trials, accelerating timeline by 25%.
- •Implemented an innovative project management framework that reduced overhead costs by $1.2 million over five projects.
- •Managed stakeholder communications for a key clinical project, resulting in increased transparency and project support.
- •Conducted regular progress reviews and status updates, maintaining a project completion rate of 95% on schedule.
- •Devised a risk assessment strategy that improved early risk identification and mitigation, reducing potential project disruptions by 30%.
- •Collaborated with regulatory teams to ensure 100% compliance with FDA guidelines during drug development phases.
- •Managed budgeting and resource planning for three major drug development projects, under-budget by 15% cumulatively.
- •Coordinated a multidisciplinary team for effective communication, leading to an 18% improvement in project delivery times.
- •Developed a comprehensive project plan that streamlined operations and improved efficiency across the board.
- •Successfully led a project reinforcing compliance with EMA standards, ensuring zero regulatory discrepancies.
- •Presented project progress to executive leadership, resulting in a 20% increase in stakeholder engagement and confidence.
- •Supported project managers in executing biotech projects by providing detailed analysis of project performance metrics.
- •Facilitated problem-solving sessions, leading to a 15% reduction in project delays.
- •Created resource allocation plans that maximized team efficiency, resulting in 10% faster project delivery.
- •Provided documentation and compliance support, ensuring all projects adhered to the outlined regulatory frameworks.
- •Assisted in managing project schedules and timelines, maintaining an on-track completion rating for all assigned tasks.
- •Coordinated cross-functional team meetings to enhance collaborative project execution and improved outcomes.
- •Maintained the project management software database, ensuring accurate and up-to-date project information.
- •Supported senior project managers by tracking project expenses and assisting with budget reallocation processes.
Pharmaceutical Project Planning Manager resume sample
- •Spearheaded a team of 10 to develop project plans that resulted in a 25% reduction in timeline delays.
- •Implemented a resource allocation strategy, boosting project completion rates by 20% across five major projects.
- •Conducted risk management analyses which mitigated potential disruptions by 30% through innovative problem-solving.
- •Facilitated cross-functional meetings, enhancing team collaboration and reducing communication gaps by 40%.
- •Revamped strategic planning processes, improving efficiency and resulting in a 15% reduction in project durations.
- •Steered budget management that achieved cost savings of approximately $500,000 across various projects.
- •Coordinated with regulatory teams to ensure 100% compliance with GMP standards, enhancing project legitimacy.
- •Optimized project reporting tools, decreasing reporting time by 15% and increasing accuracy in data presentation.
- •Led a multifaceted initiative that increased process transparency and reduced stakeholder inquiries by 30%.
- •Mentored junior project managers, improving departmental efficiency by 20% over a year-long mentorship program.
- •Orchestrated resource allocation meetings, aligning team efforts with project metrics and improving project deliverables.
- •Developed and monitored project timelines, contributing to on-time completion of 90% of projects.
- •Organized stakeholder communication frameworks, improving project transparency and stakeholder satisfaction by 25%.
- •Assisted in budget preparation leading to 5% cost savings over projections in fiscal year 2016.
- •Facilitated project lifecycle phases ensuring adherence to project milestones, maintaining a 95% on-track rate.
- •Assisted in preparing regulatory documentation, ensuring 100% compliance for FDA submissions.
- •Collaborated with cross-functional teams to streamline processes, achieving faster approval times for over 10 applications.
- •Evaluated regulatory data, increasing report accuracy by 20% and enhancing submission quality.
- •Supported project teams in managing timelines effectively, boosting adherence rates to 98% over one year.
Quality Assurance Project Manager in Pharma resume sample
- •Directed cross-functional teams in quality audits, identifying compliance gaps and increasing success rates by 35% over two years.
- •Implemented new quality metrics that increased regulatory compliance by 25%, enhancing overall product integrity.
- •Developed and executed quality assurance strategies and plans, resulting in reduced production errors by 22% within a year.
- •Coordinated with R&D and Regulatory teams to integrate quality standards, boosting project execution efficiency by 18%.
- •Enhanced corrective and preventive action protocols, reducing operational risks and achieving a 20% improvement in process efficiency.
- •Spearheaded external audits, achieving zero critical findings across three separate audits over 12 months.
- •Managed multiple QA projects simultaneously, maintaining 98% on-time delivery through effective project scheduling and resource management.
- •Enhanced quality assurance processes, leading to a 40% increase in audit compliance rates through proactive risk assessments.
- •Implemented continuous improvement initiatives, improving product quality metrics by 15% over a 24-month period.
- •Collaborated with operations to optimize batch review processes, reducing review times by 20% and increasing throughput.
- •Guided project teams on regulatory requirements, effectively minimizing compliance issues and enhancing team knowledge by 30%.
- •Led internal quality audits, addressing findings promptly and reducing repeat issues by 35% through effective CAPA strategies.
- •Integrated new quality management systems, streamlining processes and reducing documentation errors by 28%.
- •Conducted extensive risk assessments, identifying potential compliance risks and implementing mitigations reducing them by 21%.
- •Trained 50+ staff members in GMP regulations, significantly improving operational compliance and understanding across teams.
- •Assisted in the development of quality assurance strategies leading to a 20% increase in product quality consistency.
- •Participated in cross-functional meetings, ensuring quality integration, thereby enhancing project outcomes by 15%.
- •Supported quality audits, contributing to 100% first-pass audit success for the team’s projects over two years.
- •Designed and maintained detailed quality documentation, improving accessibility and accuracy of information by 25%.
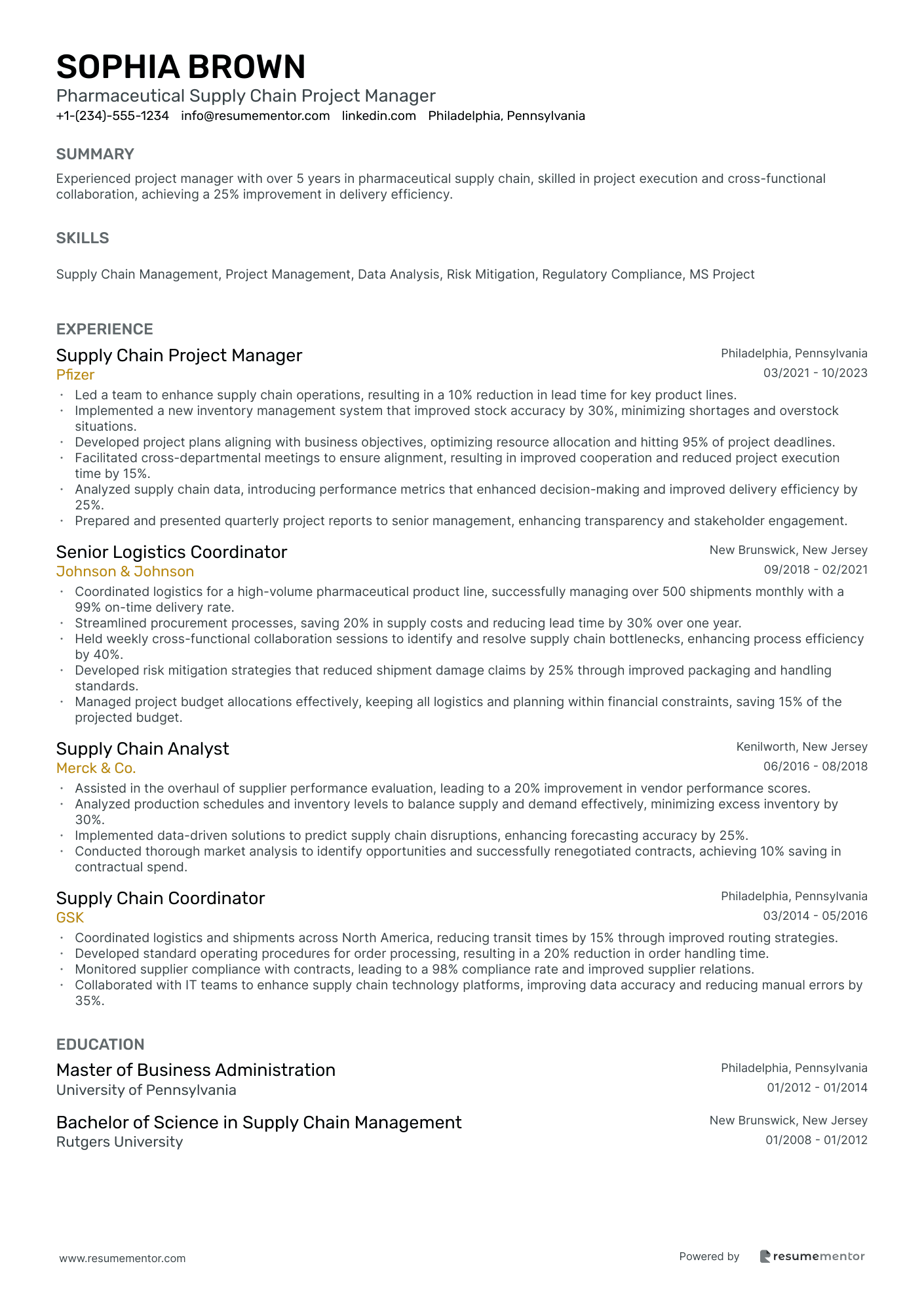
Pharmaceutical Supply Chain Project Manager resume sample
- •Led a team to enhance supply chain operations, resulting in a 10% reduction in lead time for key product lines.
- •Implemented a new inventory management system that improved stock accuracy by 30%, minimizing shortages and overstock situations.
- •Developed project plans aligning with business objectives, optimizing resource allocation and hitting 95% of project deadlines.
- •Facilitated cross-departmental meetings to ensure alignment, resulting in improved cooperation and reduced project execution time by 15%.
- •Analyzed supply chain data, introducing performance metrics that enhanced decision-making and improved delivery efficiency by 25%.
- •Prepared and presented quarterly project reports to senior management, enhancing transparency and stakeholder engagement.
- •Coordinated logistics for a high-volume pharmaceutical product line, successfully managing over 500 shipments monthly with a 99% on-time delivery rate.
- •Streamlined procurement processes, saving 20% in supply costs and reducing lead time by 30% over one year.
- •Held weekly cross-functional collaboration sessions to identify and resolve supply chain bottlenecks, enhancing process efficiency by 40%.
- •Developed risk mitigation strategies that reduced shipment damage claims by 25% through improved packaging and handling standards.
- •Managed project budget allocations effectively, keeping all logistics and planning within financial constraints, saving 15% of the projected budget.
- •Assisted in the overhaul of supplier performance evaluation, leading to a 20% improvement in vendor performance scores.
- •Analyzed production schedules and inventory levels to balance supply and demand effectively, minimizing excess inventory by 30%.
- •Implemented data-driven solutions to predict supply chain disruptions, enhancing forecasting accuracy by 25%.
- •Conducted thorough market analysis to identify opportunities and successfully renegotiated contracts, achieving 10% saving in contractual spend.
- •Coordinated logistics and shipments across North America, reducing transit times by 15% through improved routing strategies.
- •Developed standard operating procedures for order processing, resulting in a 20% reduction in order handling time.
- •Monitored supplier compliance with contracts, leading to a 98% compliance rate and improved supplier relations.
- •Collaborated with IT teams to enhance supply chain technology platforms, improving data accuracy and reducing manual errors by 35%.
Navigating the job market as a pharmaceutical project manager can feel like trying to develop a new drug without a clear formula. Crafting the perfect resume is a common headache. You might struggle with articulating your skills, experience, and achievements in a way that stands out in the crowded pharmaceutical industry. A lack of industry-specific resume templates can make your job search even more difficult. Hiring managers are looking for specific qualifications that prove you can handle the unique demands of pharmaceutical projects. Without the right resume, you risk getting lost in the shuffle.
To make your journey smoother, it’s crucial to use a resume template tailored for pharmaceutical project managers. A specialized template will highlight your most relevant skills and experiences, making a strong first impression on hiring managers. The more closely your resume aligns with industry expectations, the higher your chances of landing that dream job.
We have over 700 resume examples that you can use to create a standout resume.
Key Takeaways
- Tailor your pharmaceutical project manager resume using a specialized template to highlight relevant skills and experiences, and increase your chances of making a strong first impression on hiring managers.
- Include key sections such as Contact Information, Professional Summary, Work Experience, Skills, Education, Certifications, and optionally "Projects" and "Awards" to showcase specific achievements and recognitions.
- Showcase your experience with project management tools, budget management, product launches, and relevant certifications like PMP, emphasizing achievements and measurable outcomes to stand out.
- Use a reverse-chronological format, modern fonts like Rubik and Montserrat, and save your resume as a PDF to maintain formatting and compatibility with Applicant Tracking Systems (ATS).
- Highlight both hard skills (e.g., Clinical Trials Management, Regulatory Compliance) and soft skills (e.g., Leadership, Problem-solving) to ensure a comprehensive representation of your qualifications.
What to focus on when writing your pharmaceutical project manager resume
Your pharmaceutical project manager resume should show your experience in managing complex projects, your ability to ensure compliance with regulatory standards, and your skill in coordinating cross-functional teams. Highlight your achievements in successfully bringing products to market, managing budgets, and meeting deadlines. Make sure to include your proficiency in project management tools and any relevant certifications.
- Use of project management software like MS Project or Asana
- Details of project budgets managed and outcomes achieved
- Examples of successful product launches or regulatory approvals
- Certifications like PMP (Project Management Professional)
Must have information on your pharmaceutical project manager resume
To create a standout pharmaceutical project manager resume, it’s essential to include key sections that highlight your expertise and qualifications. Make sure your resume includes:
- Contact Information
- Professional Summary
- Work Experience
- Skills
- Education
- Certifications
Adding sections like "Projects" and "Awards" can further enhance your resume, showcasing specific achievements and recognitions in your field. These can help you stand out to potential employers.
Which resume format to choose
For a pharmaceutical project manager resume, use a reverse-chronological format to showcase your experience effectively. Fonts like Rubik and Montserrat are modern and professional alternatives to Arial and Times New Roman. Always save your resume as a PDF to maintain formatting.
Set your margins to one inch to ensure readability and compatibility with Applicant Tracking Systems (ATS). Use clear section headings like "Professional Experience" and "Education," as these help ATS parse your information correctly.
Important sections for a pharmaceutical project manager resume include:
- Contact Information
- Professional Summary
- Skills
- Professional Experience
- Education
- Certifications
- Technical Skills
- Projects
Resume Mentor's free resume builder handles all of this, making your resume creation stress-free and professional.
How to write a quantifiable resume experience section
When writing the experience section of your resume as a pharmaceutical project manager, you should be clear and concise. Present your work history in reverse-chronological order, starting with your most recent job. Aim to include roles from the past 10-15 years, highlighting key positions relevant to the job you're applying for. Tailoring your resume for the specific role you want is crucial. This means using action words and focusing on achievements rather than generic responsibilities.
You want to balance between being brief and informative. Use action words like "led," "achieved," and "managed" to demonstrate impact. Numbers and specific achievements will make your experience stand out. Let's explore two examples to show a poorly written experience section and an outstanding one.
Here is the first example that is badly written:
- •Responsible for ensuring projects stayed on schedule
- •Managed a team of 5 members
- •Interacted with stakeholders frequently
This example is bad because it lacks specifics and impact. It primarily describes responsibilities, which are vague and not quantifiable. There's no emphasis on achievements, making it hard for potential employers to see the value you added.
Now, let's look at the awesome example:
- •Led cross-functional teams to deliver 15+ drug development projects on time and within budget
- •Increased project efficiency by 25% through streamlined processes and innovative solutions
- •Secured $5M in funding by building compelling project proposals and presenting to investors
This example is good because it uses action words and quantifiable achievements. It clearly demonstrates the impact of your role, showing you led teams, increased efficiency, and secured funding. This approach makes your experience tangible and compelling to potential employers.
Pharmaceutical project manager resume experience examples
Ready to boost your career? Creating the perfect resume is a "p(h)arm"-ful task, but worry not! Here’s a dose of well-tailored resume experience sections specifically for pharmaceutical project managers—get ready to cure your CV woes!
Achievement-focused
When focusing on achievements, highlight measurable outcomes. This demonstrates your capacity to deliver significant results.
Pharmaceutical Project Manager
HealthMed Innovations
January 2020 - Present
- Increased project efficiency, reducing time to market by 20%
- Achieved $10 million in annual revenue within the first quarter post-launch
- Received recognition for exceptional project management excellence
Skills-focused
Showcasing your skills can be as powerful as outlining your achievements. Ensure to highlight those most relevant to pharmaceutical project management.
Project Manager
BioPharma Corp
June 2018 - December 2019
- Utilized Agile methodologies for streamlined development processes
- Leveraged risk management tools to preempt project challenges
- Applied data-driven decision making to optimize resource allocation
Responsibility-focused
Detailing responsibilities gives potential employers a clear idea of what you handled in previous roles.
Pharmaceutical Project Lead
Innovative Pharma Solutions
March 2017 - May 2018
- Coordinated activities among regulatory, clinical, and marketing teams
- Implemented project timelines, ensuring adherence to budgets
- Maintained compliance with industry-specific regulations
Project-focused
Highlight specific projects you’ve managed to demonstrate your ability to see projects through successfully.
Senior Project Manager
NextGen Pharmacy
April 2015 - February 2017
- Managed a budget of $15 million, ensuring financial efficiency
- Led a team of 50+ professionals across different disciplines
- Achieved full regulatory approval ahead of schedule
Result-focused
Results speak volumes about your capabilities. Emphasize the tangible results of your efforts.
Project Manager
Prime Pharmaceutics
January 2013 - March 2015
- Streamlined manufacturing processes to enhance efficiency
- Negotiated with suppliers to reduce material costs by 15%
- Improved team productivity through targeted training programs
Industry-Specific Focus
Tailor your experience to highlight your expertise in the pharmaceutical industry.
Pharmaceutical Project Manager
OncoMed Pharmaceuticals
July 2010 - December 2012
- Led clinical trials in collaboration with top-tier medical institutions
- Ensured compliance with FDA and EMA guidelines
- Developed and maintained project documentation for regulatory audits
Problem-Solving focused
Showcase your ability to navigate challenges and come up with effective solutions.
Project Manager
Global Pharma Solutions
September 2008 - June 2010
- Identified bottlenecks in the supply chain and implemented solutions
- Established alternative sourcing strategies to mitigate delays
- Improved communication channels between departments for faster problem resolution
Innovation-focused
Emphasize your role in driving innovation within pharmaceutical projects.
Pharmaceutical Project Manager
TechPharm Innovations
February 2006 - August 2008
- Spearheaded the integration of AI to predict project risks and outcomes
- Enhanced data analysis capabilities to optimize project performance
- Received industry accolades for innovative project management strategies
Leadership-focused
Leadership qualities are crucial for a project manager; underline your leadership experience.
Project Lead
EndoPharma
October 2004 - January 2006
- Fostered a collaborative team environment to boost morale and productivity
- Mentored team members, facilitating professional development
- Implemented leadership training programs to enhance team capabilities
Customer-focused
In pharmaceuticals, focusing on customer needs can be a game-changer; highlight your customer-centric projects.
Pharmaceutical Project Manager
ClientCare Pharma
May 2002 - September 2004
- Developed extensive customer feedback systems to improve products
- Collaborated with marketing teams to understand customer needs
- Implemented customer feedback into product development cycles
Growth-focused
Demonstrate your role in driving company growth through successful project management.
Project Coordinator
GrowthHealth Corp
February 2000 - April 2002
- Identified new markets for pharmaceutical products
- Expanded project pipelines to increase company revenue
- Fostered partnerships with key industry stakeholders
Efficiency-focused
Projects in the pharmaceutical industry must be efficient. Highlight how you’ve streamlined processes.
Pharmaceutical Project Manager
EfficienPharm
January 1998 - January 2000
- Implemented Lean Six Sigma methodologies
- Optimized resource allocation through predictive analytics
- Established performance metrics to continuously monitor efficiency
Technology-focused
Technology is crucial in modern pharmaceuticals. Emphasize your role in tech integration.
Project Manager
PharmaTech Solutions
June 1996 - December 1997
- Utilized project management software to increase oversight and control
- Adopted cloud-based solutions for real-time collaboration
- Implemented automated reporting systems to enhance data accuracy
Collaboration-focused
Effective collaboration is key in pharmaceutical projects. Highlight your cooperative efforts.
Project Facilitator
CollaborativePharm
April 1994 - May 1996
- Coordinated efforts between R&D, marketing, and regulatory teams
- Facilitated regular cross-functional meetings to align project goals
- Encouraged knowledge sharing to drive project success
Training and Development focused
Highlighting training initiatives demonstrates your commitment to team growth and development.
Project Manager
Pharmaco Developments
January 1992 - March 1994
- Created a comprehensive onboarding program for new hires
- Facilitated ongoing training workshops to upskill team members
- Tracked progress and adjusted training methods to ensure effectiveness
Write your pharmaceutical project manager resume summary section
When writing your resume summary section as a pharmaceutical project manager, it's crucial to focus on clarity and relevance. This section should immediately inform employers about your professional experience and key skills. A summary section generally comprises three to four concise sentences that encapsulate your career achievements and what you bring to the table.
Here’s a bad example:
This example lacks specifics and comes off as generic. Employers won’t see how you stand out or what exact value you bring.
Now, here’s a good example:
This example is strong because it highlights specific achievements and metrics. It tells employers the exact value you add, with measurable success.
Always be sure to tailor your summary to the job you're applying for. Highlight the most relevant skills and experiences to catch the employer’s eye.
What you say about yourself in a resume summary sets the tone for the rest of your resume. Use clear and specific language. Mention your key skills and experiences but don't overinflate your abilities. Honesty and precision are essential.
A resume summary differs from a resume objective, profile, and summary of qualifications in subtle ways. A resume objective focuses on your career goals. A resume profile is a more detailed account of your skills and experiences. A summary of qualifications lists bullet points of your key accomplishments and skills.
Use your resume summary to give a brief overview of your professional experience and the value you bring. Make it powerful, concise, and relevant to the position you're seeking.
Listing your pharmaceutical project manager skills on your resume
Writing the skills section for your pharmaceutical project manager resume can make a significant difference in catching the attention of hiring managers. Skills can be highlighted in a standalone section, or implemented within other parts of your resume like the summary and experience sections. Strengths and soft skills refer to your personal attributes such as leadership, communication, and problem-solving abilities. Hard skills are specific, teachable abilities, such as proficiency with project management software or regulatory knowledge.
Skills and strengths serve as crucial resume keywords, improving your chances of passing through applicant tracking systems (ATS) and drawing the eyes of hiring managers to your qualifications.
A standalone skills section allows for a quick scan of your most relevant abilities. Here's an example:
This skills section is effective because it lists specific abilities relevant to a pharmaceutical project manager role. Each skill is clear and professional, covering essential job functions that employers look for. The list is concise, making it easy for hiring managers to quickly identify your qualifications.
Best hard skills to feature on your pharmaceutical project manager resume
A successful pharmaceutical project manager must have a variety of hard skills to manage and lead projects efficiently. These skills should communicate your technical knowledge and ability to handle industry-specific tasks.
Hard Skills
- Clinical Trials Management
- Regulatory Compliance
- Budget Management
- Risk Management
- Quality Assurance
- Data Analysis
- Project Planning
- Research Methodology
- Gantt Chart Proficiency
- MS Project
- Statistical Software Proficiency
- Change Management
- Drug Development Process
- SOP Writing
- Product Lifecycle Management
Best soft skills to feature on your pharmaceutical project manager resume
Soft skills are equally important for a pharmaceutical project manager. They demonstrate your ability to work well with others and drive projects to successful completion.
Soft Skills
- Leadership
- Communication
- Problem-solving
- Time Management
- Team Collaboration
- Adaptability
- Conflict Resolution
- Decision-making
- Attention to Detail
- Critical Thinking
- Emotional Intelligence
- Negotiation
- Multitasking
- Stress Management
- Interpersonal Skills
How to include your education on your resume
An education section is an essential part of your resume as a pharmaceutical project manager. It's crucial to tailor this section to the job you're applying for, including only relevant education while omitting what's not pertinent. If your GPA is noteworthy (typically above 3.5), it's a good idea to include it. Mention honors like "cum laude" to highlight your academic excellence. Listing your degree should follow a consistent format with the degree first, followed by the institution, location, and dates of attendance.
The first example is poorly written because it includes irrelevant education. An associate degree in graphics design is not useful for a pharmaceutical project manager role.
The second example is excellent because it includes a relevant degree. Listing a Bachelor of Science in Pharmaceutical Sciences demonstrates your academic preparation for the role. Including the "cum laude" honor and a 3.8 GPA highlights your success and dedication, making this section strong and relevant.
How to include pharmaceutical project manager certificates on your resume
Including a certificates section in your pharmaceutical project manager resume is crucial. Certifications can endorse your skills and validate your expertise. You can incorporate the certificates section in the header. For instance, add "PMP Certified | Lean Six Sigma Black Belt" directly under your name.
List the name of each certificate. Include the date when you received it. Add the issuing organization to authenticate the credential. Ensure the certificates are relevant to pharmaceutical project management to show your qualification for the role.
This example is effective because it includes highly relevant certifications for the role. Both certificates align well with the essential skills required for a pharmaceutical project manager. The details provided, such as the issuing organizations, underscore the legitimacy and prestige of the credentials. This approach enhances your resume's impact, making you a more compelling candidate.
Extra sections to include in your pharmaceutical project manager resume
In the dynamic world of pharmaceuticals, a project manager must skillfully balance scientific knowledge, commercial acumen, and leadership abilities. Your resume needs to reflect these diverse skills, but also highlight your unique attributes beyond just professional capabilities.
Language section — Show how multilingual skills allow for better communication with international teams and stakeholders. Highlighting language proficiency can demonstrate your capability to navigate global projects.
Hobbies and interests section — Illustrate how interests, like hiking or cooking, reveal your personality and stress-management techniques. This section can make your resume more relatable and engaging for HR.
Volunteer work section — Underscore volunteer experiences that develop leadership and project management skills. Things like managing a charity run or organizing community health events can reflect your commitment to both leadership and public health.
Books section — Mention books that have influenced your professional growth or mindset, such as "The Lean Startup" by Eric Ries or "Good to Great" by Jim Collins. This can showcase your continual learning and thought process in managing pharmaceutical projects effectively.
Pair your pharmaceutical project manager resume with a cover letter
A cover letter is a brief document that you send with your resume when applying for a job. It explains why you are interested in the position and how your skills and experiences make you the best fit. This letter helps you introduce yourself in a more personalized way, showcasing your enthusiasm and professionalism.
For a pharmaceutical project manager, the cover letter should focus on your experience managing clinical trials, your knowledge of regulatory compliance, and your skills in team leadership and project planning. Highlight any successful projects you have managed, including their outcomes and how they benefited the company. Mention specific technical skills or certifications you hold that are relevant to the job.
Now is the perfect time to create your cover letter using Resume Mentor's cover letter builder. It's easy to use and generates a professional document in minutes. Plus, the PDF export feature helps protect your content and formatting, ensuring your letter looks polished and professional. Start now and take the next step in landing your dream job!
Henry Jackson
San Antonio, Texas
+1-(234)-555-1234
help@resumementor.com
Related Articles

Continue Reading
Check more recommended readings to get the job of your dreams.
Resume
Resources
Tools
© 2026. All rights reserved.
Made with love by people who care.

